Living in the Era of the Fontan: Failure Should Simply Not be an Option
Last Updated: September 09, 2022
Evolution of the Fontan
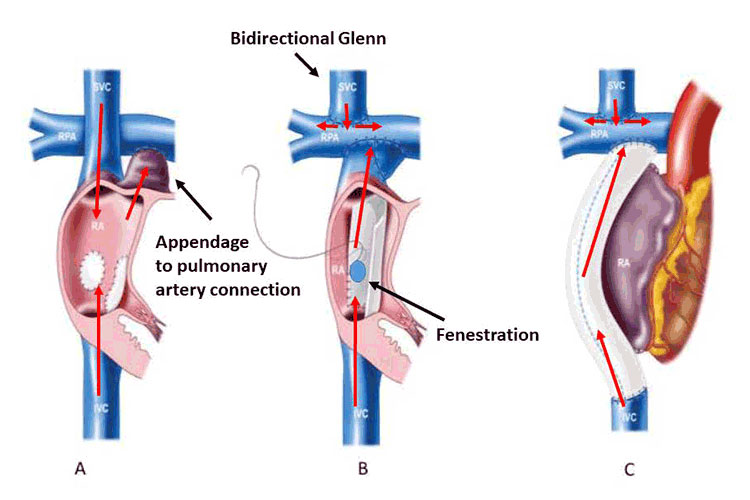
Staged single ventricle palliation and the Fontan operation have undergone many iterations over the past five decades. It was originally thought that atrial systole was essential to propel blood forward, thus the entire right atrium was incorporated into the circuit with the appendage anastomosed to the pulmonary artery, atriopulmonary connection (APC) Fontan; Figure 1A. This eventually resulted in severe right atrial dilation, with large energy losses impeding forward flow. These patients face a high burden of complications including atrial arrhythmias and thrombus formation. Today, the total cavopulmonary anastomosis is completed in two stages. A hemi-Fontan or bidirectional Glenn operation (Figure 1B), performed around age 3-6 months, directs superior vena caval flow into the pulmonary arteries. The inferior caval anastomosis and completion of the Fontan then occurs in early childhood (typically between 2-4 years old). Many of these patients will have also undergone neonatal palliative procedures to establish stable sources of pulmonary and systemic blood flow. In the 1980s it was recognized that first performing the Glenn operation allowed the heart more time to adjust to being volume offloaded and improved short-term survival.3 The modern day Fontan is performed either as a lateral tunnel or extra-cardiac conduit (Figures 1B, 1C), with an ongoing debate over which of these is superior in terms of hemodynamic efficiency and long-term cardiac complications.4 Preference for Fontan type is generally center-specific, as is whether to routinely create a fenestration at surgery (Figure 1B). Fenestrations have demonstrated early post-operative benefits (decreased incidence of pleural effusions and shorter post-operative hospitalization) at the expense of mild cyanosis. There is no clear long-term benefit to maintaining a patent fenestration and there is a theoretical risk, albeit small, for paradoxical venous thromboembolism.5 For patients who maintain a persistent fenestration into adulthood, there is uncertainty regarding the indications for fenestration closure and the best regimen for thromboprophylaxis. As the authors of the AHA Statement point out, there are ongoing efforts to further optimize the Fontan circuit, with a particular recent interest in utilizing a modified Y-graft for the inferior caval connection. This has the theoretical benefit of improved fluid dynamics and more evenly distributed hepatic blood flow to the lungs, which may prevent the formation of pulmonary arteriovenous malformations due to a yet-to-be identified “hepatic factor.” Thus far, this modification has not demonstrated superiority. In the cohort of APC style Fontan patients who experience significant cardiac complications, conversion to an extracardiac conduit (often combined with a maze procedure) may be considered. However, early mortality after Fontan conversion is as high as 10%, particularly in those patients referred late and with more advanced Fontan failure.6
Over time, patient selection for Fontan surgery has also evolved. In 1977, Chouset outlined his famous “10 Commandments” for selecting Fontan candidates.7 With the evolution of modern surgical and interventional techniques, many of the original commandments have undergone modification. However, they remain relevant by highlighting the guiding principles for selecting appropriate candidates for single ventricle palliation. Criteria include anatomy that is surgical amenable to incorporation into a Fontan circuit, low resistance in the pulmonary vascular bed and Fontan circuit, atrioventricular synchrony and reasonable systemic ventricular function. A recent systematic review found that systemic ventricular dysfunction and atrioventricular valve regurgitation were the most significant factors contributing to death or urgent heart transplantation.8 In patients at high-risk for Fontan completion, directly proceeding to transplantation may be a preferred alternative. Evidence suggests that Glenn physiology serves as a more stable bridge to transplant and early Fontan failure is associated with higher post-transplant mortality.9 Traditionally, the majority of patients undergoing pre-Fontan evaluation have received cardiac catheterization to confirm suitability. However, with advances in non-invasive imaging modalities, specifically cardiac MRI, some centers have moved toward entirely non-invasive means of assessing candidacy for low-risk patients. A single center study of Fontan patients who received pre-surgical assessment with cardiac MRI compared to cardiac catheterization found no significant difference in short-term outcomes.10
Life of the Fontan Patient
Among the most significant contributions of the AHA Statement is in providing an outline of the various sequelae experienced by palliated single ventricle patients. Due to underlying genetic abnormalities and prolonged cyanosis affecting neurodevelopment, learning and behavioral difficulties are frequently encountered. Rates of anxiety and depression are extremely high in adolescent and adult Fontan patients and the provider needs to be keen toward their identification. Most Fontan patients have impaired exercise tolerance, and it is important to encourage physical activity and dispel incorrect beliefs about activity limitations. Obesity rates have climbed significantly in the Fontan population, perhaps more rapidly than in the general population; and evidence suggests that routinely active Fontan patients may experience near normal exercise tolerance.11 Female Fontan patients may desire pregnancy; and in absence of high-risk features, this is often feasible, albeit with an increased risk for cardiovascular complications (including arrhythmia and heart failure). Women considering child bearing should see a maternal fetal medicine specialist and have extensive discussion of risk before pregnancy. Infertility and miscarriage rates are strikingly high, and patients should understand that maternal life expectancy is inherently limited. The Fontan circulation also creates a prothrombotic state, which when combined with a frequent burden of atrial arrhythmias creates a high risk for clot formation. All Fontan patients should be maintained on some form of thromboprophylaxis. In the absence of atrial arrhythmia or prior thrombus history, no significant difference has been seen in thrombus prevention comparing aspirin and full-dose anticoagulation with warfarin.12 Though direct oral anticoagulants (DOACs) are gaining popularity, there is insufficient safety and efficacy data to recommend their routine use. Although the list of potential comorbidities can be daunting, more often than not, Fontan patients maintain excellent quality of life for decades and enjoy living independently, working and forming strong relationships.
The Failing Fontan
As the AHA Statement discusses, the single ventricle patients enter into a form of chronic heart failure from the time of Fontan operation. In this unique physiologic state, cardiac output is determined not by systemic ventricular function, but rather by the impedance to blood flow.1 Both systolic and diastolic ventricular dysfunction are frequently seen; and standard adult heart failure pharmacotherapies are frequently employed despite little evidence supporting their efficacy in Fontan patients. Elevated pulmonary vascular resistance (PVR) plays a critical role in the failing Fontan, with even mild elevations in PVR resulting in detrimental effects. Initial studies of advanced medical therapies for pulmonary hypertension in failing Fontan patients have shown modest improvements in exercise capacity and heart failure symptoms, but it remains unclear which patients derive the most benefit.13 The combination of chronically reduced cardiac output and elevated venous pressures results in a number of systemic complications, the gravest of which is protein-losing enteropathy (PLE). Historically, PLE carried a 50%, 5-year mortality. In the modern era this has improved to 88% and 72% at 5 and 10 years, respectively.14 The pathophysiology of PLE is multifactorial and remains incompletely characterized, though recent evidence suggests lymphatic congestion and spillage may play a central role, as it also does in plastic bronchitis (PB), another serious Fontan complication. Lymphatic embolization may prove to be an effective treatment for both PLE and PB, but further study is necessary. The development of fibrotic liver disease is universal in Fontan patients and routine screening for Fontan-associated liver disease (FALD) and hepatocellular carcinoma as outlined in the “Fontan Circulation Surveillance Testing Toolkit” is of the utmost importance, particularly given that FALD is often clinically silent until advanced stages of cirrhosis.
Transplantation
Although Fontan palliation has revolutionized the care of single ventricle patients by creating a stable circulation that can last decades, it is the expectation that failure is an inevitability. Both patients and their families should be counseled early regarding the role of heart transplantation, with the knowledge that it too is not a cure. Given a continued shortage of donor organs and an ever-increasing population of Fontan patients, creating viable forms of mechanical circulatory support (MCS) for the Fontan patient is critical. Within the pediatric transplant population, one in five children is now bridged to transplant with a ventricular assist device. Their use has led to significant improvements in end-organ perfusion and has cut wait-list mortality in half.15 However, use of MCS in the Fontan population has thus far demonstrated limited success.16 The AHA Statement summarizes the current issues facing MCS use in this population, including highlight on the questionable benefit in Fontan failure with preserved systolic function.
Although the potential need for transplantation is always present, it remains one of the most controversial areas of Fontan care. Patients are often not referred until they have experienced clinical deterioration that results in significant end organ dysfunction that may ultimately prohibit being listed. There may also be trepidation on the part of the transplant team that the patient is “too complex” or “too high risk.” A high burden of panel reactive antibodies is also frequently present following multiple prior surgeries and blood product exposure. For those Fontan patients ultimately listed for transplant, additional barriers include lower priority status, longer wait times and ultimately a higher waitlist mortality.17 One-year transplant outcomes in pediatric Fontan patients are excellent and equal to non-congenital patients. Data evaluating transplant outcomes in adult Fontan patients remains limited. Studies of the adult congenital population at large have demonstrated higher mortality within the first year post transplant but better long-term survival compared to non-congenital patients.18 Reported single center experiences of transplant survival in Fontan patients indicates worse short-term outcomes compared to other forms of congenital heart disease.
Defined criteria to guide timing of transplant listing remain elusive. Worsening functional status by serial measurement, deteriorating ventricular function and elevation in serum cardiac biomarkers are all associated with worsened survival for adult congenital heart failure patients, but the significance of individual data points or the absolute change between measures has not been defined. Cardiopulmonary exercise test is one of the most powerful tools for determining transplant listing eligibility in the non-congenital adult population, but it remains unclear which exercise measures are the most meaningful in Fontan patients and whether a cut-off value or overall trend provides better prognostic information.19 The indications for multi-organ transplant, predominantly combined heart-liver transplant, are equally murky. However, there is a growing body of experience with combined heart-liver transplantation, and early experiences suggest favorable outcomes.20
Conclusions
The Writing Group should be commended for their extensive efforts in highlighting the significant progress achieved in the treatment of single ventricle heart disease over the previous five decades, as well illustrating the remaining challenges in caring for this population. Attempts are being made to standardize the care and surveillance of Fontan patients, and the Surveillance Toolkits are an excellent starting point. Continued multi-center collaboration will undoubtedly be critical to the continued growth of evidence-based management in this patient population.
Figure 1
Variations of Fontan anatomy: Classic atriopulmonary connection (A), lateral tunnel (B) and extracardiac conduit (C). Direction of venous blood flow is illustrate by red arrows. Modified from d’Udekem Y, et al. The Fontan Procedure. Circulation. 2007;116[suppl I]:I-157-I-164.)
Citation
Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, Hsia T-Y, Hsu DT, Kovacs AH, McCrindle BW, Newburger JW, Pike NA, Rodefeld M, Rosenthal DN, Schumacher KR, Marino BS, Stout K, Veldtman G, Younoszai AK, d’Udekem Y; on behalf of the American Heart Association Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Council on Cardiovascular and Stroke Nursing. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association [published online ahead of print July 1, 2019]. Circulation. doi: 10.1161/CIR.0000000000000696.
References
- Gewillig M, Goldberg DJ. Failure of the fontan circulation. Heart Fail Clin. 2014;10(1):105-116.
- Schilling C, Dalziel K, Nunn R, et al. The Fontan epidemic: Population projections from the Australia and New Zealand Fontan Registry. Int J Cardiol. 2016;219:14-19.
- Jonas R. Intermediate Procedures After First-Stage Norwood Operation Facilitate Subsequent Repair. Ann Thorac Surg. 1991;52:696-700.
- Khairy P, Poirier N. Is the extracardiac conduit the preferred Fontan approach for patients with univentricular hearts? The extracardiac conduit is not the preferred Fontan approach for patients with univentricular hearts. . 2012;126(21):2516-2525; discussion 2525.
- Atz AM, Travison TG, McCrindle BW, et al. Late status of Fontan patients with persistent surgical fenestration. J Am Coll Cardiol. 2011;57(24):2437-2443.
- Brida M, Baumgartner H, Gatzoulis MA, Diller GP. Early mortality and concomitant procedures related to Fontan conversion: Quantitative analysis. Int J Cardiol. 2017;236:132-137.
- Stern HJ. Fontan "Ten Commandments" revisited and revised. Pediatr Cardiol. 2010;31(8):1131-1134.
- Kulkarni A, Patel N, Singh TP, Mossialos E, Mehra MR. Risk factors for death or heart transplantation in single-ventricle physiology (tricuspid atresia, pulmonary atresia, and heterotaxy): A systematic review and meta-analysis. J Heart Lung Transplant. 2019.
- Kovach JR, Naftel DC, Pearce FB, et al. Comparison of risk factors and outcomes for pediatric patients listed for heart transplantation after bidirectional Glenn and after Fontan: an analysis from the Pediatric Heart Transplant Study. J Heart Lung Transplant. 2012;31(2):133-139.
- Brown DW, Gauvreau K, Powell AJ, et al. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional Glenn anastomosis: long-term follow-up of a prospective randomized trial. J Thorac Cardiovasc Surg. 2013;146(5):1172-1178.
- Cordina R, du Plessis K, Tran D, d'Udekem Y. Super-Fontan: Is it possible? J Thorac Cardiovasc Surg. 2018;155(3):1192-1194.
- Marrone C, Galasso G, Piccolo R, et al. Antiplatelet versus anticoagulation therapy after extracardiac conduit Fontan: a systematic review and meta-analysis. Pediatr Cardiol. 2011;32(1):32-39.
- Clift P, Celermajer D. Managing adult Fontan patients: where do we stand? Eur Respir Rev. 2016;25(142):438-450.
- John AS, Johnson JA, Khan M, Driscoll DJ, Warnes CA, Cetta F. Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J Am Coll Cardiol. 2014;64(1):54-62.
- Zafar F, Castleberry C, Khan MS, et al. Pediatric heart transplant waiting list mortality in the era of ventricular assist devices. J Heart Lung Transplant. 2015;34(1):82-88.
- Adachi I, Burki S, Fraser CD, Jr. Current Status of Pediatric Ventricular Assist Device Support. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2017;20:2-8.
- Alshawabkeh L, Opotowsky AR, Carter KD, et al. Disparities in Wait-List Outcomes for Adults With Congenital Heart Disease Listed for Heart Transplantation Before and Since Revision of Status I Listing. Am J Cardiol. 2018;122(10):1761-1764.
- Bradley EA, Pinyoluksana KO, Moore-Clingenpeel M, Miao Y, Daniels C. Isolated heart transplant and combined heart-liver transplant in adult congenital heart disease patients: Insights from the united network of organ sharing. Int J Cardiol. 2017;228:790-795.
- Menachem JN, Reza N, Mazurek JA, et al. Cardiopulmonary Exercise Testing-A Valuable Tool, Not Gatekeeper When Referring Patients With ACHD for Transplant Evaluation. World J Pediatr Congenit Heart Surg. 2019:2150135118825263.
- Vaikunth SS, Concepcion W, Daugherty T, et al. Short-term outcomes of en bloc combined heart and liver transplantation in the failing Fontan. Clin Transplant. 2019:e13540.
Science News Commentaries
-- The opinions expressed in this commentary are not necessarily those of the editors or of the American Heart Association --
Pub Date: Monday, Jul 01, 2019
Author: Jordan D. Awerbach, MD, MPH & Richard A. Krasuski, MD
Affiliation: Division of Cardiovascular Medicine, Duke University Medical Center, Durham, N.C.